The Challenge
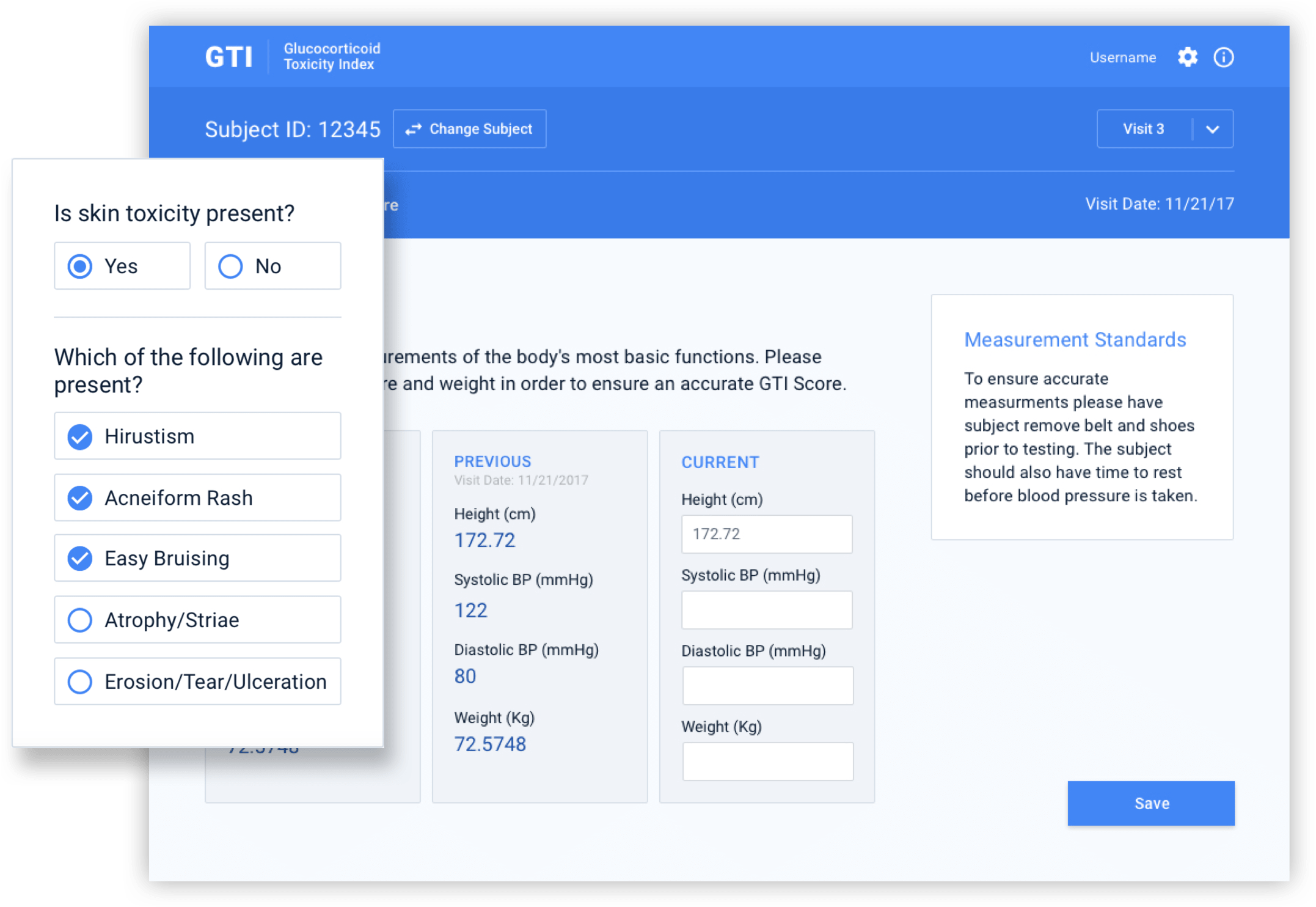
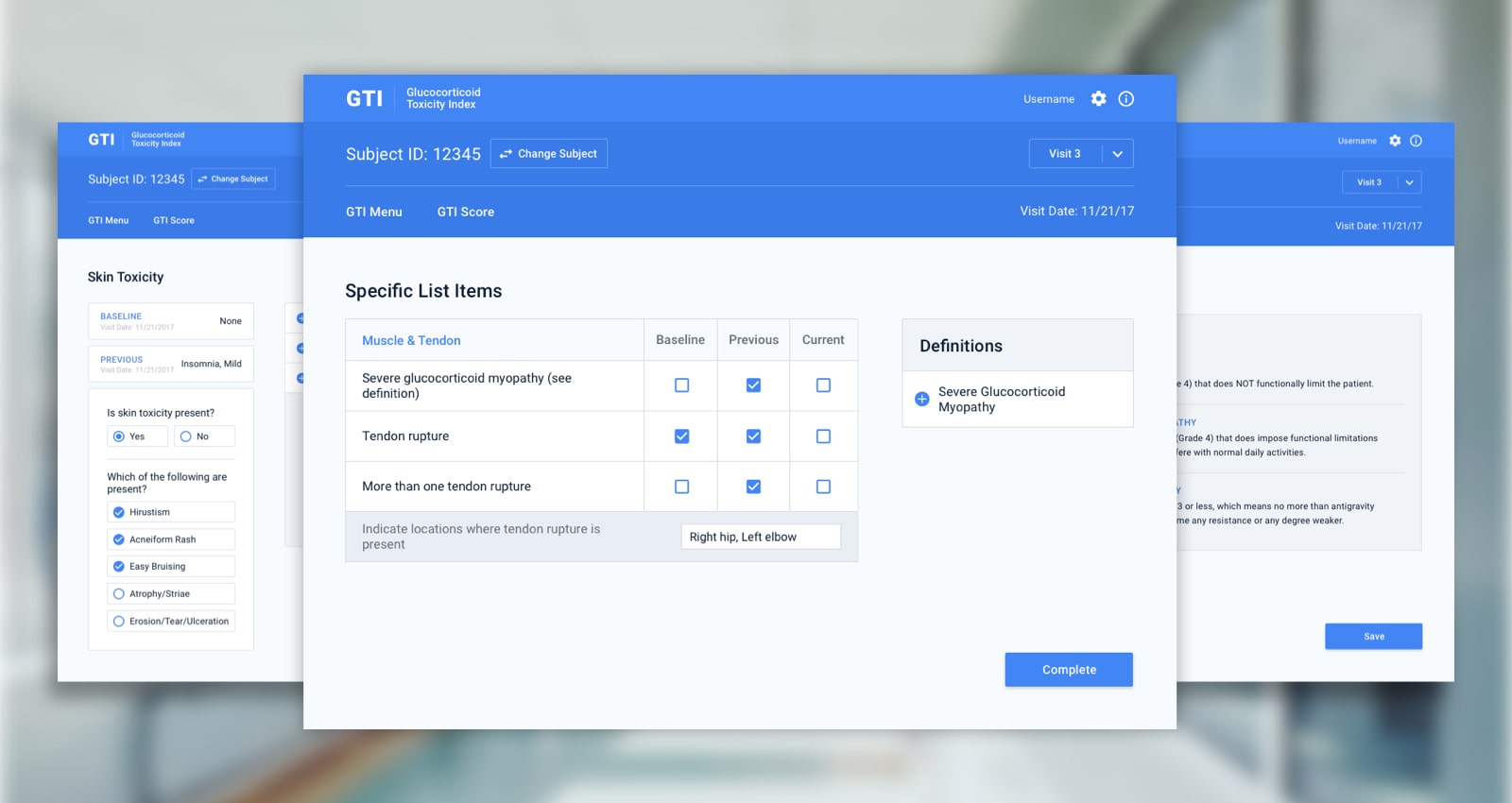
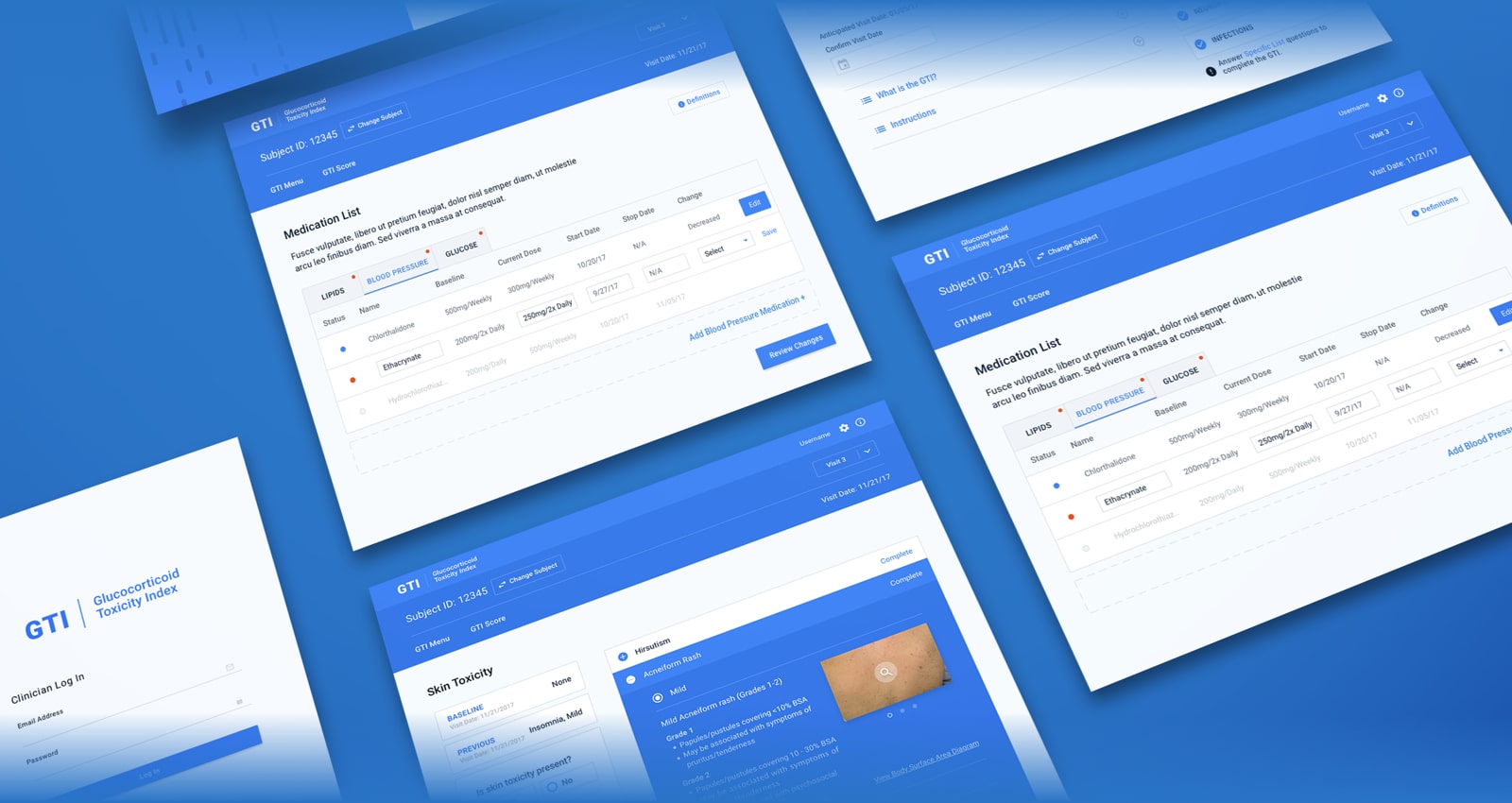
The pen and paper administration of a breakthrough steroid toxicity measurement tool made its use in clinical trials difficult, hampering research organizations and pharmaceutical companies.
Dr. John Stone, MD, MPH and Director, Clinical Rheumatology at Massachusetts General Hospital, and his colleague, Dr. Eli Miloslavsky, recognized a major flaw in steroid toxicity measurements in clinical trials. To address this problem, they developed the Glucocorticoid Toxicity Index (GTI), which is able to measure changes in steroid toxicity over time.
Initially, the tool was administered in clinical trials via pen and paper, but this method was inefficient and limited research capabilities. The doctors knew they needed a more scalable, researcher-friendly solution that would appeal to research organizations and pharmaceutical companies for use in clinical trials.